India recorded its first Covid19 case on the fateful day of January 30th, 2020.
According to sources, first detected when a medical student residing in Wuhan, China, returned to India.
On March 25th last year, Prime Minister Narendra Modi, due to the rising number of cases of Covid-19, announced a nationwide lockdown.
The COVID-19 pandemic has left the world with shattered hopes. Schools and colleges shut down, malls and movie halls were closed, life as we knew it completely changed.

Fast forward to December 2020, after months of lockdown, curfews, and living in uncertainty finally, we saw hope in the form of Covid-19 vaccines. After relentless efforts, various medical labs, and organizations, developed few vaccine prototypes which were in the advanced stages of human trials.
HOW DOES THE COVID-19 VACCINE WORK?
The COVID-19 vaccines develop immune responses to the SARS-Cov-2 virus and thereby protect us from the disease.
If individuals get immunity, the chances of spreading the disease will also become narrow, thereby protecting the community.
IS THE VACCINE SAFE?
In this turbulent and chaotic time, where nothing has been guaranteed, we often find ourselves asking if the vaccine is, after all, safe and effective. Here’s a brief description of the stages of trial that the vaccines go through before they are marked safe for public use.
Like all vaccines, COVID-19 vaccines are going through a rigorous, multi-stage testing process, including extensive (phase III) trials that involve thousands of people. These trials, which include some groups at high risk for COVID-19 (excluding certain groups like pregnant and lactating women in vaccine trials), are specifically designed to identify any common side effects or other safety concerns. It is imperative to protect people at increased risk for severe illness from COVID-19, such as healthcare providers, elderly individuals, and people with other medical conditions.

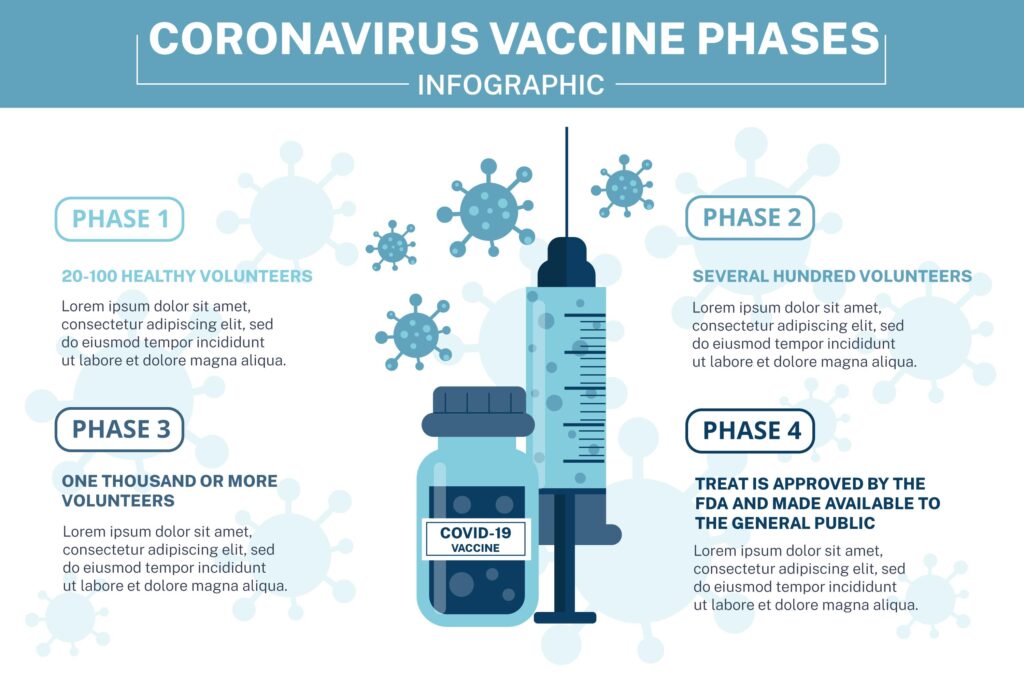
PHASES OF CLINICAL TRIAL: We must monitor the safety and efficacy of the vaccine under various tests and trials before it can be labeled safe for mass use.
- PRE-CLINICAL TEST:
Before the first clinical test, it is usually administered on mice, rabbits, and pigs to observe the reaction to the vaccine.
- PHASE 1:
After passing the pre-clinical test, the vaccine is tested on a small group of people to know if it negatively affects the immune system.
- PHASE 2:
In the second phase of the trial, an extensive group of people (nearly 100 individuals divided into subgroups) was vaccinated to pass another safety test.
- PHASE 3:
In the third phase, also known as the efficacy test, they used the vaccine on thousands of people.
However, even though the vaccine might prevent you from getting adversely ill, it’s not yet confirmed that it will reduce your chances of spreading the disease. Hence social distancing, sanitization, and masks will remain a part of our journey for the foreseeable future.